Deoxyribonucleic acid (DNA) is a double-helical macromolecule. With two sugar-phosphate backbones on the outside, it is comprised of repeated stacks of either adenine (A)–thymine (T) or guanine (G)–cytosine (C) base pairs, coupled by hydrogen bonds(Watson–Crick model).
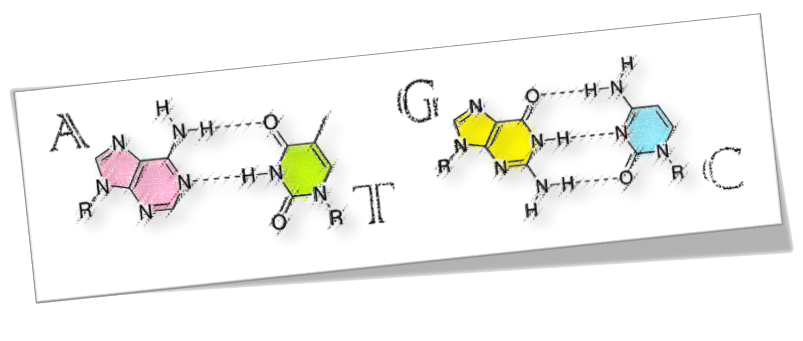
DNA is the carrier of genetic information and the foundation material of biological heredity. Besides, sequence-specific DNA, which is distinct in any living organism, virus or pathogen, can be detected based on the hybridization with its complementary strand. DNA possesses complementarity, self-assembling and self-recognition abilities that always form hydrogen-bonded base pairs where the base pairs line up one-dimension-ally at 0.34–0.36 nm intervals.
A key structural feature, which makes DNA a promising candidate for applications in nanometer-scale electronics, is the array of π-stacked base pairs. As a functional nano-size material, DNA possesses properties of both nano-scale templates and molecular wires. Taking advantage of properties of DNA and integrating them with current semiconductor technology could lead to the development of nano-scale electronic devices, including the biosensor.[ref.1]
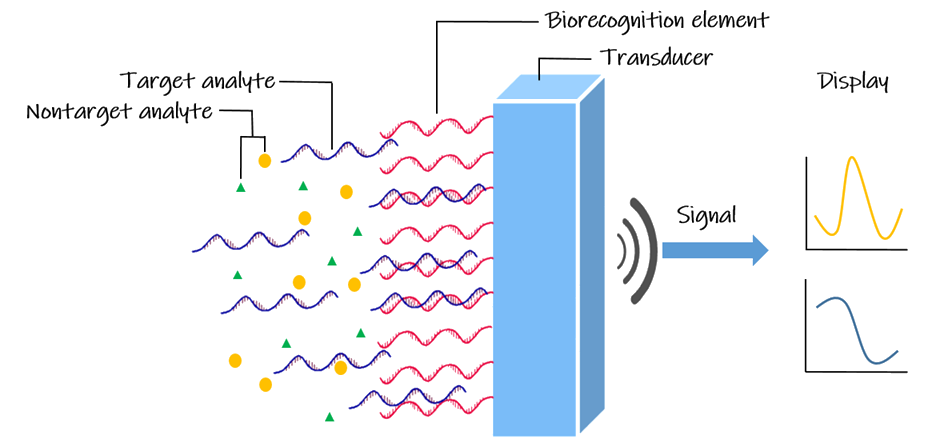
Coupling a biological recognition element that interacts with the target analyte and a transduction element, DNA biosensors are used to obtain the DNA sequence’s specific information. In other words, it has a wide range of applications, including the DNA-based diagnosis and pathogen detection. Electrochemical DNA-based biosensors have been widely developed due to the remarkable advantages of easy operation, rapid response, less power requirement, miniaturized platform and low cost. The DNA-electrochemical biosensor is formed by an electrode (the electrochemical transducer) with a DNA probe (the biological recognition element) immobilized on its surface and is used to detect DNA-binding molecules (the analyte).[ref.4,5]
The World Health Organization(WHO) defines superbugs as microorganisms that develop antimicrobial resistance. [ref.6] The mechanism by which superbugs develop resistance to antibiotics is basically controlled by genes, collectively referred to as drug resistance genes. Some bacteria are inherently resistant to genes and may be transmitted via plastids to obtain resistance genes from other bacteria. In today's clinical medicine, antibiotics are an obvious treatment for bacterial infections, and as a result, they are medically abused. With the emergence of different antibiotics, especially the broad-spectrum antibiotics, the bacteria with the resistance genes have been forced to survive and threaten humans. In the rapid evolution process, bacteria can survive quickly by adapting to the environment, and drug resistance genes render existing antibiotics useless. These superbugs cause humans to face the great impact from medical care expenses. In the face of serious bacterial infections, the use of antibiotics is necessary, but in the environment of antibiotics, more and more drug-resistant bacteria will emerge. It leads to the dilemma in medical treatments. Therefore, how to quickly and accurately diagnose whether an individual is infected with bacteria that have a drug resistance gene and to identify its type, so that accurate drug administration can be made and thereby reduce inappropriate antibiotic use, is the key to greatly reduce the possible emergence of superbugs.
One of the most globally important microorganisms is methicillin-resistant Staphylococcus aureus (MRSA). Staphylococcus aureus is a major human and veterinary pathogen worldwide. Methicillin-resistant S. aureus poses a significant and enduring problem to the treatment of infection by such strains. MRSA has become epidemic not only in nosocomial infections but also in community-associated infections. MRSA that has been a common cause of nosocomial infections worldwide also has been on the rise in the communities in recent years. Invasive infections of MRSA have high morbidity and mortality rates. Most of invasive staphylococcal and community-acquired MRSA (CA-MRSA) infections are related to the nasal carriage of Staphylococcus. Resistance is usually conferred by the acquisition of a nonnative gene encoding a penicillin binding protein (PBP2a), with significantly lower affinity for β-lactams. This resistance allows cell-wall biosynthesis, the target of β-lactams, to continue even in the presence of typically inhibitory antibiotic concentrations. PBP2a is encoded by the mecA gene, which is carried on a distinct mobile genetic element (SCCmec), and the expression of which is controlled through a proteolytic signal transduction pathway comprising a sensor protein (MecR1) and a repressor (MecI). Many of the molecular and biochemical mechanisms underlying methicillin resistance in S. aureus have been elucidated, including regulatory events and the structures of key proteins.[ref.7,8,9,10]
The common biosensor is a single electrode, and a single electrode can only detect a DNA fragment of a probe length, about 16-30-mer, and it is impossible to determine a longer-length target DNA and a sequence that does not complement the probe, that is, it cannot be accurately judged whether the added sample is 100% of our target DNA. The two electrodes pass through as the bridge, and the probe DNA on both sides is added to the target DNA to carry an energized circuit. A complete long piece of DNA can be carried on the pier formed by the DNA probes on both sides, so that the electrodes on both sides can form a pathway. The different distances between the electrodes and different probe lengths can be adjusted with the target DNA to detect more complete long-segment target DNA under more specific conditions.

Within the timeframe of BIOMOD, it is important to make a balance between reality and ideal in our project. Our ideal goal is to make a biosensor that can detect different antibiotic resistance genes; however, for the competition, we’d like to make some strategies. And by means of these strategies, we can create a prototype first, verify the core concept of our project, and test the feasibility of our product.
Due to the limited among of time, it is hard to achieve all of the ideas mentioned above in our project. The followings are the goals we want to reach someday in the future, and the short-term goal is the primary one that we will complete before the BIOMOD.
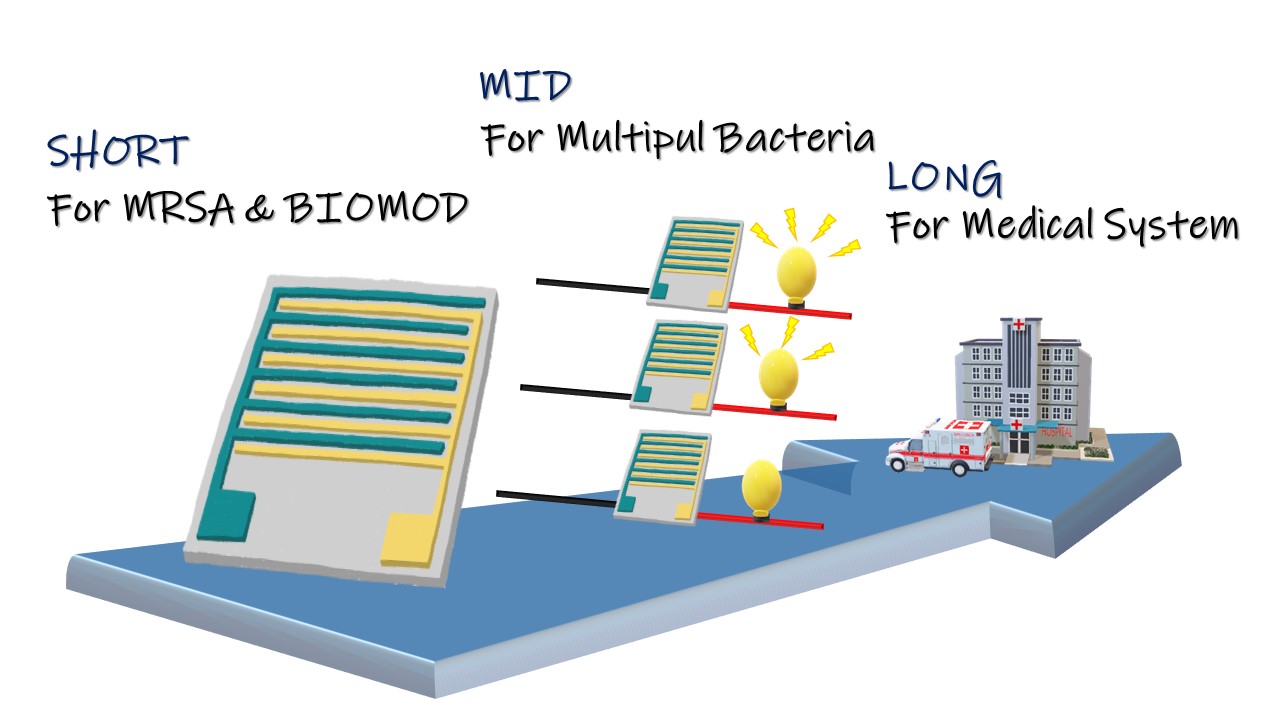
In this period, we make efforts to produce a prototype for our ideal biochip, FF, using MRSA for BIOMOD competition as well as using FF to prove our core concept and the mechanism of FF itself.
In order to improve biochip FF, we connect several biochips, which, with different capture probes in parallel, form a parallel electric circuit, so that the whole device could detect more than one antibiotic resistance gene at the same time.
Promote and commercialize our biochip FF; in addition, we’d like to push it to the developing countries and to current medical systems.
After constantly pondering, combining the fluorescent substance on DNA will be our new goal. Though there are many similar fluorescent detection assays, there are some problems about fluorescent intensity and its wavelength, causing problems like that the light is too weak or identified to be the invisible light. Because it can not be observed by eyes, we still need to use the instrument to detect it. We consider that the step of instrumental detection is most likely to break through. If we can accomplish using visible light to determine test results, like the detection probe we will mention in Design later, we can not only promote the reliability because of visual result, but have the advantage of double checking.
Separately, large quantity and diverse tests are our goal, too. We clearly know that the advantage of our products is using electric circuit to leave out complex PCR steps. Using our biochip FF can save a lot of waiting time. The design of in-parallel electric circuit is our new idea. It's only feasible by reorganizing the electric circuit, thus our design of semiconductor comes in handy. In-parallel electric circuit means we can test a lot of samples at the same time, even the targets are not only mecA fragment. For different to-detect genes, we only need to change the capture probes on the chips. Based on the above principles, we can make many chip models for different genes and connect them in parallel to achieve this future goal: detect different genes at the same time.
By incorporating mass production, our biochip will not only reduce the cost of production, but promote the popularity rate to even reach the commercial level. A simple, creative and more efficient testing way is our biggest goal. Getting better test efficiency can assist us to apply in hospitals and personal homes broadly. Separately, due to that healthcare in developing countries is not universal, inaccurate usage of medicine leads to the spread of superbug. Hence, if our biochip FF can achieve point of care testing (POCT), a form of testing in which the analysis is performed where healthcare is provided close to or near the patient, it will be the medical resource improving the medical quality in the developing countries.
After modifying the biochip FF itself, our biochip is still a monomer, though. However, if there has any possibility of combining more diverse technology products to challenge the infinitive possibilities of our biochips? After exploring farther, directing the carefully designed biochips to the emerging trend of the biotechnology field, AI robot, poses the potential. When AI robot formally goes into the medical field, the robot’s functional intensity determines its practicality. Our gene- detecting biochips will be the best tools to improve the robot’s performance. Before detecting, the pretreatment of sample will be done in the robot. This will completely develop our advantages: precision, simple, quick and inexpensive. Certainly, the advanced ideas must face more technical problems, not only electronic engineering but all medical aspects have to be solved. If the biochips FF can achieve these ideas, we believe that we can get a better answer to the question - how humans face the drug resistance genes- in the future.